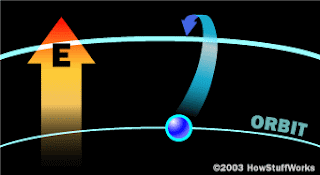
Absorbing EnergyConsider the illustration from the previous page. Although more modern views of the atom do not depict discrete orbits for the electrons, it can be useful to think of these orbits as the different energy levels of the atom. In other words, if we apply some heat to an atom, we might expect that some of the electrons in the lower-energy orbitals would transition to higher-energy orbitals farther away from the nucleus.
Once an electron moves to a higher-energy orbit, it eventually wants to return to the ground state. When it does, it releases its energy as a photon -- a particle of light.
No comments:
Post a Comment